What did they say? "Carbon dioxide emissions", "marine pollution", "deforestation"? Maybe if they thought for a little longer some might say, "the way that human beings are living their lives."
I think, on balance, I would go along with the latter, but one phrase has been haunting me since I started researching this article: "methane hydrates". Those two words may not be familiar to most people, but I am starting to realise that if I had to pick out just one specific reason to fear the future, methane hydrates would be it. I would add, though, that this selfsame knowledge, if spread widely enough, may buy us enough time to give life on Earth a fighting chance. First, though, I’m going to talk about eggs.
If I want to boil an egg I have to heat some water to boiling point, so I put some water in a saucepan and place it on my gas cooker hob. To get the energy into the water, to heat the water, to cook the egg, I turn on the gas. Then I do something magical – I create a spark and ignite the gas flowing out of the burner.
Don’t you think that’s magical? Ok, I’ll explain. In the time between turning the gas supply on and lighting the gas a stream of almost pure methane (CH4) emerged from the burner. The moment that I lit the gas, the heat from the spark started a chemical reaction that combined oxygen from the air with carbon in the methane to form carbon dioxide. The remaining hydrogen formed a hot plasma that we recognise as the (hopefully) blue flame that heats my pan of water, that cooks my egg.
My gas burners produce about 7000BTUs of energy every hour, using about 7 cubic feet of natural gas. If I had failed to light the burner for an hour, not only would I risk burning my house down, but I would produce about 198 litres of methane, with the same global warming potential as 1663 litres of carbon dioxide. With the burner lit, my 198 litres of methane is converted to about 198 litres of carbon dioxide. Simply by burning the methane rather than releasing it into the air, I have reduced the global warming potential over 100 years by a factor of 8.4. In fact, according to the IPCC’s 4th Assessment Report, over the shorter - and more relevant - period of 20 years I would have reduced the global warming potential of my methane by a factor of 26.3, just by lighting the burner. That is why you see gas flares wherever a large amount of natural gas is produced, refined or stored.
So what has this got to do with methane hydrates? Well, methane is responsible for about 20% of the greenhouse effect we are currently experiencing, so, if you would excuse me for a while, I want to tell you a little bit about where it all comes from.
Sources Of Methane
According to convention, emissions of greenhouse gases are split into those caused by humans (known as Anthropogenic) and those caused by natural processes. Unfortunately, particularly in the case of methane, this division does not tell the whole story. For a start, some causes are a combination of human and natural processes – such as forest fires, which can start spontaneously, or can be set off deliberately by humans. Also, the source of the gas may be natural, but the actual release may be triggered largely or solely by humans, and may not even be taking place at present.
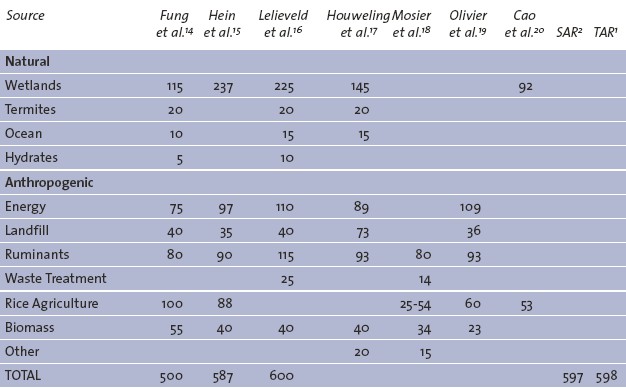
Given these difficulties, I’m going to provide a quick guide to the main sources of methane not divided into human and natural sources, but by whether the rate of emission can be significantly controlled by humans or not. For technical information I have drawn heavily on the University of Oxford’s excellent Methane UK report, and the US Environmental Protection Agency statistics. The chart at the top, showing the relative, current emissions of all the main methane sources (in millions of tonnes) is drawn from the Oxford report.
Uncontrollable Sources
By far the largest uncontrollable source of methane is wetlands, accounting for around a third of the global total. The other term for methane, Marsh Gas, derives from this. I say "uncontrollable" but this is not strictly true. Methane is produced by organisms known as Methanogenic Bacteria, under conditions devoid of oxygen. These anaerobic conditions make the difference between the decomposition of organic matter producing methane, and otherwise producing the far more benign carbon dioxide under aerobic conditions. Humans can have an impact on the amount of methane being released through a range of direct and indirect processes.
One direct process is the drainage of wetlands which initially causes a sudden "exhalation" of methane, but then has the effect of reducing the long term release given that the wetland is drying out or no longer exists. Another process, indirect in this case, is the warming of the wetland environment which, at between 37 and 45 degrees Celsius, maximises methane production. It is almost inevitable, given the current warming trend, that more tropical wetlands will fall into this bracket in the future.
The jury, though, is out on the long term impact of these and other influences on wetland methane, so for now we shall say that approximately a third of the current methane supply is probably out of our control.
Back in 1989, a New Scientist article highlighted the role that termites play in methane emissions. At the time it was thought that only around 1% of methane emissions were caused by the digestive processes of the 250 trillion termites on Earth, but now the figure is thought to be around 3% of the total – still small, definitely natural and almost certainly out of our control.
The final uncontrollable source is that produced by bacteria in the oceans. Little is known about the way this can vary or what precisely causes it, but as it is only responsible for around 2% of the total emissions then it can safely be put in the "uncontrollable" camp.
Controllable Sources
There are lots of controllable sources; many of them easily controllable, some which can be controlled but not always for the best, and one which we are in danger of losing control of entirely. In all, the controllable sources make up around two thirds of all the methane being emitted into the atmosphere at present.
Energy sources of methane make up around 25% of the controllable emissions. 65% of this figure comes from natural gas, which can be lost in its unburnt form anywhere in the extraction, refining, transportation and use stages. Naturally, the main (direct) culprits are those countries that process natural gas, with the USA and Russia being responsible for about 30% of the total natural gas emissions. Prevention is surprisingly simple – plug the leaks in the transmission system and responsibly flare off any excess gas, although it would be far preferable to use all of the gas extracted or, better still, not extract the gas in the first place.
Not so simple is controlling that other significant portion resulting from coal mining. China is responsible for 40% of coal mining emissions, a figure that is expected to grow. But the western world is not without blame; not only is Europe and the USA responsible for another 40% of this figure, but there are no accurate records of the amount of methane escaping from abandoned coal mines, or which there are many. As both our consumption of natural gas, and especially coal continues to rise, methane emissions will certainly increase – especially in parts of the world with poorer engineering and safety standards. The best way to reduce the emissions from coal would be to stop it being used in the first place!
Landfill is a significant, but stable source of methane, accounting for around 10% of controllable emissions. Landfill methane has been dramatically reduced in industrialised nations over the last 15 years, mainly due to the use of methane as a semi-renewable energy resource in landfill sites. But as we become an ever more wasteful society – and despite the dramatic reduction in landfill expected in the European Union over the next 10 years – the amount of rubbish we pour into the land, and the resulting methane, will keep going up. Waste water could account for as much as 10% of the total again, largely due to open sewers and lagoons allowing the organic matter to ferment. As would be expected, non-industrialised countries, especially China and India are responsible for the bulk of these, technically easy to reduce, emissions.
One of the most controversial topics around methane is that of ruminant emissions. Specifically, methane comes from two main sources, the waste material (manure) that is stored and used as a valuable organic fertilizer, and that which is emitted directly from – largely – cattle, known as Enteric Fermentation. Manure only accounts for about 2-3% of the controllable total, and is fairly easily controlled by using sealed storage and combustion containers. Animal emissions – which largely come out of the front, rather than the back end – are responsible for up to 30% of all controllable methane emissions, or 6% of all anthropogenic greenhouse gases. This figure will, again, keep rising as a result of increased global population and also the "westernisation" of diets around the world. Not surprisingly, the main sources are those countries with large cattle populations – Brazil, China and India. The solution is simple, if we are prepared to change our diets radically in favour of non-animal products, but it doesn’t look as though that going to happen anytime soon.
Biomass combustion is a story of two parts, both of which are eminently preventable. With around 3% of the total preventable emissions, wood burning predominantly produces methane in non-industrialised countries. The methane derives from the incomplete consumption of the organic matter in the wood, something that can be easily solved by using more efficient stoves. The larger portion (7-8%) is, sadly, a result of forest burning, with the biggest contributors being Brazil, Indonesia, the Democratic Republic of Congo and many other central African countries. Again, the methane is released with the incomplete burning of organic matter, and with the loss of rainforest comes a dismal tale of both methane and carbon dioxide release, and the loss of irreplaceable habitat – usually for the purpose of clearing land for farming. Much of that farming involves cattle rearing.
Combining both farming and wetlands, is the staple crop of a third of the world’s population. Rice agriculture is only a source of methane when carried out in a flooded, or paddy, state. With 90% of rice cultivated in this way, not only are water demands huge, but methane emissions are disproportionate compared to the actual energy content of the crop. The emissions are not easily calculated, largely due to constantly changing rice farming conditions, but they may account for as much as 25% of all controllable methane emissions, with China and India producing the lion’s share. There is certainly a strong case for using alternating wet-dry methods of agriculture, and possibly increases in salt-water paddies, but unless a massive and possibly more damaging change to dry-field crops takes place then paddy rice will continue to be a methane source we have to work with rather than remove altogether.
Some potentially significant sources of methane have recently been disclosed, but are not included in official figures due to uncertainty; these include the highly controversial – but possibly not relevant - discovery that plants may emit large amounts of methane and also the production of methane by methanogenic bacteria in artificial reservoirs. This latter source could be easily controlled either by removing the methane at source, or simply removing the dams entirely – certainly a better outcome for the river systems being killed by the presence of the dams.
It should already be clear that much of the potential for reductions in methane emissions lies squarely with the industrialised world, in terms of providing the necessary expertise in reducing poorer countries’ emissions, and also ensuring that its own practices – such as the consumption of animal products, and the overuse of energy sources – are dramatically curtailed.
The last controllable source is methane hydrates, a completely natural substance that we have only just become fully aware of. Remember at the beginning of this article I said that the mere mention of methane hydrates makes my skin go clammy, or words to that effect, well we have now reached the point when I need to explain why.
Methane Hydrates
Buried deep within the permafrosts of western Siberia, safe from harm, lies something in the order of 70 billion tonnes of methane.
This methane is stored, like other vast reservoirs in the ancient frozen peat bogs, and far under the oceans, in odd structures known as Clathrates. The type of clathrates that store methane, usually known as methane hydrates, consist of molecules of solidified water inside which methane molecules are contained. Due to the high pressures under which they exist – beneath hundreds of metres of frozen peat or oceanic sediment and water – methane hydrates can contain many times more methane than could exist in the same volume of air.
It is perfectly feasible, therefore, that relatively small areas of land, like the west Siberian permafrost, or the deep sediments around the Gulf of Mexico, have enormous potential both for energy supply and heating the atmosphere to a level that would cause a catastrophic mass extinction of life.
If we take the current controllable methane sources as being about 300 million tonnes (or 6400 million tonnes carbon dioxide equivalent) then the complete release of the western Siberian reservoir would be the equivalent of 233 years of current human methane emissions. It is believed, however, that the total reservoir of methane hydrates within the permafrost and under the sea could be anything between 750 and 3000 billion tonnes of methane, or up to 2000 years worth of human greenhouse gas emissions.
Now, I’m not suggesting for a minute that all of this methane is going to pop into the atmosphere in one great Earthly gasp – there is no chance of this happening – but there is plenty of evidence to suggest that the great permafrosts of the north are no longer staying frozen during the summer months, and may be disappearing entirely. Although methane emissions have temporarily stabilised due to the drying out of wetlands, they will again begin to rise inexorably, and to these emissions will be added the methane that is coming from the hydrates that were previously safely encased in the arctic ice.
No one can put a figure on the expected increase, but this is clearly a greenhouse effect feedback that we could do without. Even if the amount of methane was only doubled, we would see the total global warming potential increase by 20%. If, due to global warming, just the west Siberian permafrost reservoir were to be released, over a period of, say, 50 years, then within 12 years the amount of methane in the atmosphere would rapidly increase to 9000 million tonnes. The impact of this would be catastrophic.
The current (2007) level of methane in the atmosphere is about 1.8 parts per million. This would increase by a factor of 20 to about 36 parts per million, resulting in the amount of human induced global warming increasing by a factor of 3.6. The simple outcome of this would be an increase in global temperatures of at least 3 degrees Celsius, with a dramatic increase in violent storms, desertification, flooding and, of course, the widespread inundation caused by sea level rise. The actual outcome would be far worse – the three degree increase would trigger a further set of feedbacks described by Mark Lynas thus:
"The end of the world is nigh. A three-degree increase in global temperature would throw the carbon cycle into reverse. Instead of absorbing carbon dioxide, vegetation and soils start to release it. So much carbon pours into the atmosphere that it pumps up atmospheric concentrations by 250 parts per million by 2100, boosting global warming by another 1.5C. In other words carbon-cycle feedbacks could tip the planet into runaway global warming much earlier than anyone had expected."
And all this from just one piece of permafrost, in just one of the two great hydrate complexes on Earth. And we haven’t even considered carbon dioxide.
Defusing The Timebomb
Guess what? This doesn’t have to happen. It really doesn’t.
There is no doubt that humanity, as a whole, has to dramatically cut the amount of carbon dioxide it produces; that is a task that has to remain the top priority for every sector of society – individuals, communities, businesses and governments. Without a dramatic reduction – some estimates put it as 80-90% - in carbon emissions in the next 30 or so years, the planet will be well on the way to the kind of tipping points so graphically described in the previous section.
What struck me when I was researching this article, though, was how quickly the concentration of methane in the atmosphere can fall when its emissions are reduced, compared to the 50-200 years taken by carbon dioxide. Remember how the methane levels remained static at 9000 million tonnes in the west Siberia permafrost example? This stabilisation was because a methane molecule only remains in the atmosphere for an average of 12 years.
According to the IPCC, if we can reduce our emissions of methane by just 50% - which seems perfectly reasonable given the incredibly wasteful lives we currently lead – then within 12 years, atmospheric methane levels will have fallen to 70% of their current level. This is equivalent to a 5.4% reduction in total radiative forcing (the measure of how much extra we are warming the atmosphere) - something that with carbon dioxide would take around a hundred years even if emissions were massively, and rapidly reduced.
So there you have it. A greenhouse gas that could potentially make this planet uninhabitable in a very short time is also a gas that – because we are currently producing it at pointlessly high levels - could easily be reduced with a few simple, if significant, changes, and buy us valuable time in which to fix the problem we have created.
What would you do?
(originally published at
 http://earth-blog.bravejournal.com/entry/22611)
http://earth-blog.bravejournal.com/entry/22611) 
 Homepage:
Homepage:
Comments
Display the following 2 comments